Synthesis and characterization of layered metal sulfates containing M II3(μ3-OH/F)2(M = Mg, Co) diamond chains†
Abstract
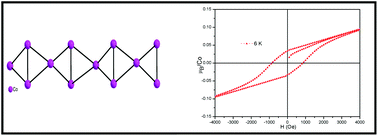
Cobalt and magnesium sulfates of the compositions, [C4N2H12]2[Co3F2(SO4)3(H2O)2], (1) and [NH4]2[Mg3(OH)2(SO4)3(H2O)2], (2), respectively, have been synthesized under hydro/solvothermal conditions, and are characterized by IR spectra, elemental analysis, powder X-ray diffraction (PXRD), energy-dispersive X-ray spectroscopy (EDX), thermogravimetric analysis (TGA), and X-ray single-crystal diffraction. 1 and 2 crystallized in the orthorhombic space groups Pnma and Cmc2 respectively. While 1 is templated by the organic piperazinium cation, 2 is obtained in the presence of ammonium ions. The layered structures are formed by the diamond chains comprising of M3(μ3-OH/F)2 units (M = Co: 1, Mg: 2). Magnetic studies of 1 reveal its ferromagnetic nature with a transition at 10.8 K and show it does not exhibit spin-glass freezing. Isothermal magnetization shows a hysteresis loop at 2.5 K with a coercive field of 1200 Oe and remnant magnetization of 0.1μB. A sharp λ-like anomaly is also seen in the heat capacity curve, favoring long range magnetic ordering below Tc.


 Please wait while we load your content...
Please wait while we load your content...