Variable coordination and C–S bond cleavage activity of N-substituted imidazolidine-2-thiones towards copper: synthesis, spectroscopy, structures, ESI-mass and antimicrobial studies†
Abstract
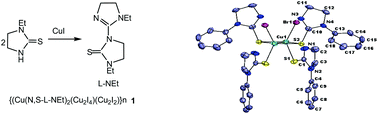
An equimolar reaction of copper(I) iodide with N-ethyl-imidazolidine-2-thione (L-Et) in acetonitrile formed black prismatic crystals over a period of three weeks. The X-ray structure determination of black crystals revealed that the thio-ligand L-Et has transformed into a new thio-ligand, 1-ethyl-3-(1-ethyl-4,5-dihydro-1H-imidazol-2-yl)imidazolidine-2-thione (L-NEt), through C–S rupture/C–N bond formation which was coordinated in the unusual 2D polymer, {(CuII(κ2-N,S-L-NEt)2)·(CuI2I4)·(CuI2I2)}n1. An ESR spectrum supported the presence of divalent CuII in the polymer. In contrast, reaction of copper(I) iodide with N-phenyl-imidazolidine-2-thione (L-Ph) in 1 : 1 molar ratio yielded a polymer, [–Cu(μ-I)2Cu(μ-S-L-Ph)2Cu–]n2, with alternate Cu2I2 and Cu2S2 cores. Further, a series of reactions of copper(I) halides with N-substituted imidazolidine-2-thiones, namely, L-R in 1 : 2 metal to ligand molar ratio (M : L) in acetonitrile have yielded different type of complexes: trigonal planar {CuX(κ1-S-L-R)2, R, X : Et, I, 4; Me, I, 5; Bun, I,6; Me, Br,7; Bun, Br, 8; Me, Cl, 9; Prn, Cl, 10; Ph, Cl, 11}, dinuclear [Cu2Br2(μ-S-L-Ph)2(κ1-S-L-Ph)2] 12, tetranuclear, [Cu4I2(μ-I)2(μ-S-L-Ph)4(κ1-S-L-Ph)2] 3 and hexanuclear [Cu6Cl6(μ-S-L-Bun)6] 13. All these complexes have been characterized using analytical data, IR and proton NMR spectroscopy, ESI-mass studies and single crystal X-ray crystallography. The antimicrobial activity of these complexes has been investigated against Gram positive bacteria, namely, Staphylococcus aureus (MTCC740), methicillin resistant Staphylococcus aureus (MRSA), Gram negative bacteria, Klebsiella pneumoniae (MTCC109), Salmonella typhimurium (MTCC741) and Candida albicans (MTCC227)- a yeast. It is significant to add that among various complexes tested for cytotoxicity toward living cells, 4, 5, 8, 9 and 10 complexes were toxic, while complex 12 was non-toxic.


 Please wait while we load your content...
Please wait while we load your content...