Diglycolamide-functionalized poly(propylene imine) diaminobutane dendrimers for sequestration of trivalent f-elements: synthesis, extraction and complexation†
Abstract
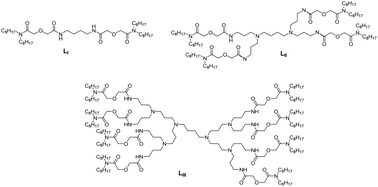
Three diglycolamide-functionalized poly(propylene imine) diaminobutane dendrimers, viz., zero generation (LI), first generation (LII), and second generation (LIII), were synthesized and evaluated for their complexation ability towards trivalent actinides and lanthanides. The distribution coefficient (D) of Am3+ with 1.0 mmol L−1 ligand in 3 M HNO3 follows the order: 0.1 (LI) < 42 (LII) < 110 (LIII). Slope analysis indicated the stoichiometry of the extracted complexes as: 1 : 2 (M : L) for LI and 1 : 2 (M : L) for both LII and LIII. A complexation study of a representative lanthanide (Eu3+) with the three ligands by absorption spectroscopy and luminescence spectroscopy confirmed the formation of the above stoichiometry. The stability constants (log β) for the ML complexes of Eu3+ with the three ligands were determined to be 5.2 ± 0.03, 5.3 ± 0.01, and 5.4 ± 0.02 for LI, LII, and LIII, respectively. The identical log β values (within experimental error) for all the ligands indicate that the complexation is not affected by the branching of the ligands. The metal/ligand complex formation was explained with the help of spectroscopic data.


 Please wait while we load your content...
Please wait while we load your content...