Exploiting the photocatalytic activity of gold nanoparticle-functionalized niobium oxide perovskites in nitroarene reductions†
Abstract
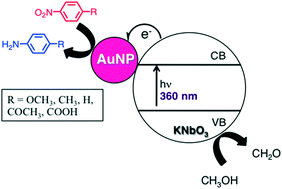
Novel gold nanoparticle-doped niobium perovskites are synthesized using a chemical reduction technique, affording supported nanoparticles on the order of 8–10 nm. The UVA-initiated photocatalytic activity of the nanocomposites was studied using nitroarene reduction as a probe reaction which yielded the corresponding aminoarenes in as little as 3 hours with yields up to 92%. Analyses of kinetic data, using Hammett parameters and computational methods, show that electron-withdrawing groups accelerate the photocatalytic process and suggest that electron transfer from the gold nanoparticle surface to the nitro group is an integral part of the rate-limiting step of this reaction. The linearity of the Hammett plot shows that no change in the photocatalytic mechanism can be expected upon variation of the para-aryl substitution. Investigation of the reaction mechanism using CH3OH-d4 illustrates that CH3OH is the most likely source of protons, as both electrons and protons are required for successful photoreduction. Moderate recyclability of the heterogeneous nanomaterial over three catalytic cycles is observed.


 Please wait while we load your content...
Please wait while we load your content...