Enhanced activity of desilicated Cu-SSZ-13 for the selective catalytic reduction of NOx and its comparison with steamed Cu-SSZ-13†
Abstract
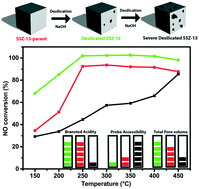
Mesoporous Cu-SSZ-13 was created by first synthesizing zeolite H-SSZ-13 and subsequently desilicating the material by base leaching using NaOH in different concentrations. The catalyst materials were prepared by ion exchanging the leached samples back to their acidic form using NH4NO3, and to their active Cu form by ion exchanging them with CuSO4. For comparison, H- and Cu-SSZ-13 were steamed using a wide variety of different conditions. Using a 0.10 M NaOH solution for base leaching, it was found that Cu-SSZ-13 becomes more active in the selective catalytic reduction of NOx with NH3 (NH3-SCR) over the entire temperature region but especially in the low temperature region (<200 °C). This increase could be explained by a decrease in pore diffusion limitations due to the introduction of mesopores on the outside of the zeolite crystals but keeping the chemical environment of the catalyst nearly the same as that of the parent material. Higher base leaching concentrations do, however, lead to a decrease in the amount of Brønsted acid sites, pore volume and accessible surface area, accompanied by a decrease in NH3-SCR activity. Ar physisorption coupled with SEM and confocal fluorescence microscopy in combination with two differently sized fluorescent organic probe molecules (i.e., 4-(4-dimethyl-aminostyryl)-1-methyl-pyridinium-iodide and 4-(4-dicyclohexyl-aminostyryl)-1-methyl-pyridinium-iodide) show an increase in the external surface area due to the creation of mesopores. The development of mesoporosity starts from the crystal surface and continues into the crystal with increasing alkaline solution strength, but under our conditions it never reaches the center. On the other hand, zeolite steaming did not successfully introduce mesoporosity and mainly managed to deactivate the Cu-SSZ-13 zeolite catalysts.


 Please wait while we load your content...
Please wait while we load your content...