CuCr2O4 derived by the sol–gel method as a highly active and selective catalyst for the conversion of glycerol to 2,6-dimethylpyrazine: a benign and eco-friendly process†
Abstract
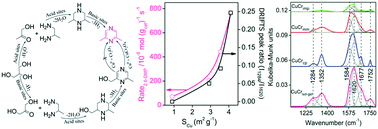
Vapour phase dehydrocyclization of crude glycerol in conjunction with 1,2-propanediamine (1,2-PDA) was examined over CuCr2O4 obtained by different preparation methods. A high proportion of copper species interacted with Cr2O3 in CuCr2O4 derived from the sol–gel route with a low ratio of Cu2+/Cu0 demonstrating higher dehydrocyclization activity and 2,6-dimethylpyrazine (2,6-DMP) selectivity. X-ray photoelectron spectroscopy analysis of the reduced CuCr2O4 revealed a lower fraction of ionic Cu and a high percentage of metallic Cu in the near surface region. The HCOOH and pyridine adsorbed DRIFT spectra of CuCr2O4 revealed that strong basic and moderate Lewis acid sites are responsible for the selective formation of 2,6-dimethylpyrazine which is consistent with the catalyst poisoning studies on CuCr2O4 co-feeding with pyridine as both Brønsted and Lewis acid site blocker and 2,6-lutidine as a selective Brønsted acid site blocker during the dehydrocyclization reaction. The presence of isolated CuO and Cr2O3 species led to a high selectivity for 2,6-dimethylpiperazine. The high intrinsic activity of CuCrsol–gel was also concomitant with the Cu metal surface areas of the catalysts. The fresh, reduced and some of the used catalysts are characterized by BET – surface area, powder XRD, FTIR, XPS, TEM, H2-TPR, TPD of NH3, pyridine, 2,6-dimethylpyridine and HCOOH adsorbed DRIFT spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...