Chromium-substituted hematite powder as a catalytic material for photochemical and electrochemical water oxidation†
Abstract
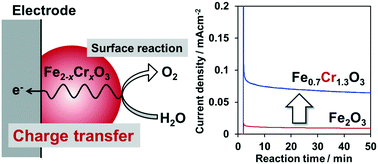
Fe(III) and Cr(III) mixed oxide (Fe2−xCrxO3) particles were examined as water oxidation catalysts under photochemical and electrochemical conditions. Photochemical water oxidation activity was evaluated under visible light irradiation (480 < λ < 500 nm) in the presence of tris(2,2′-bipyridyl)ruthenium(II) sulfate and sodium persulfate, which served as a redox photosensitizer and a sacrificial electron acceptor, respectively. Electrochemical water oxidation was conducted in a phosphate buffered aqueous solution (pH 7.5) using Fe2−xCrxO3 particles, which were loaded on a transparent conductive glass support, in the presence of an electrochemical bias. While hematite (α-Fe2O3) is known to be a catalytic material for water oxidation, its catalytic activity dropped upon substitution of Cr(III) ions for Fe(III) sites in Fe2O3 at least in part due to oxidative dissolution of Cr(III) on the oxide surface, which competed with water oxidation during the reaction. On the other hand, Cr-substitution resulted in a five-fold enhancement in the activity for electrochemical water oxidation to form oxygen. Electrochemical impedance measurements revealed that the Cr-substitution decreased the resistance of charge transfer inside the Fe2O3 bulk, thereby leading to improved performance for electrochemical water oxidation.


 Please wait while we load your content...
Please wait while we load your content...