Enzyme and photoredox sequential catalysis for the synthesis of 1,3-oxazine derivatives in one pot†
Abstract
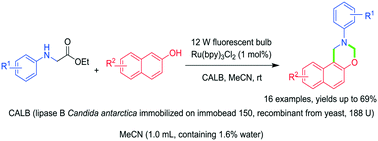
A novel strategy combining visible-light and enzyme catalysis in one pot for the synthesis of 1,3-oxazine derivatives from α- or β-naphthols and ethyl N-aryl glycinates is described for the first time. Various 1,3-oxazine derivatives were prepared with yields of up to 69% under mild reaction conditions by a simple operation. This approach consists of sequential enzymatic hydrolysis and visible-light excited decarboxylation of ethyl N-aryl glycinates, oxidation of α-amino radicals, Mannich reaction, transimination and intramolecular cyclization. This work provides a novel alternative method for the synthesis of 1,3-oxazine derivatives.


 Please wait while we load your content...
Please wait while we load your content...