Ag containing porous Au structures as highly selective catalysts for glycolate and formate†
Abstract
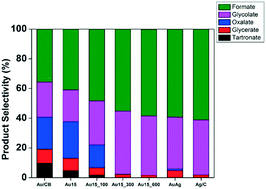
Formic acid and its salts are important chemical intermediates. Industrial production of formic acid involves liquid phase carbonylation of methanol to methyl formate followed by hydrolysis to formic acid. Alternatively, formic acid can be generated during glycerol electro-oxidation when glycerol undergoes C–C bond cleavage. Silver based catalysts such as carbon supported silver–gold alloys significantly improve selectivity to formic acid during glycerol electro-oxidation. However, these catalysts tend to suffer from poor electro-chemical activity. Herein, we examined the ability of Ag containing, porous Au structures to tune the glycerol oxidation pathway towards formate and glycolate whilst maintaining high electro-catalytic activity. Our catalyst consisted of an Au rich porous framework that possessed residual amounts of Ag within its pores. When tested for glycerol electro-oxidation, the Ag containing porous Au catalyst exhibited both high selectivity towards formate and high electrochemical activity.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...