Differentiation of the C–O and C–C bond scission mechanisms of 1-hexadecanol on Pt(111) and Ru(0001): a first principles analysis†
Abstract
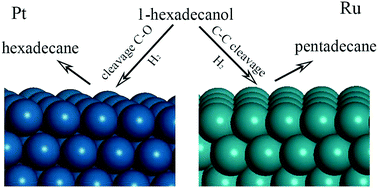
C–C and C–O bond scission are important reactions that have been extensively studied experimentally; however, the decomposition mechanism for long-chain alkanols is still not clear. In the present study, density functional theory calculations were performed to study the reaction mechanisms of C–O and C–C cleavage in 1-hexadecanol on Pt and Ru. The adsorption mechanisms and the reaction cycles for 1-hexadecanol (1-C16H34O) decomposition were clarified in this study. The mechanisms include dehydrogenation steps and C–O and C–C cleavage steps. The present calculation results show that the main mechanism of 1-hexadecanol decomposition on Pt(111) is C14H29CH2CH2OH → C14H29CH2CHOH → C14H29CH2COH → C14H29CH2C → C14H29CH2CH → C14H29CH2CH2 → C14H29CH2CH3, and the C–O bond cleavage mechanism is favored over that of C–C bond cleavage. The major final product is n-hexadecane (C14H29CH2CH3), and the C–O cleavage and the formation of the C14H29CH2CH2 species are the rate-controlling steps. However, on Ru the mechanism is C14H29CH2CH2OH → C14H29CH2CH2O → C14H29CH2CHO → C14H29CH2CO → C14H29CHCO → C14H29CH + CO → C14H29CH2 → C14H29CH3, and the dominant product is n-pentadecane (C14H29CH3). The rate-controlling steps are the dehydrogenation of C14H29CH2CO into the C14H29CHCO species and the hydrogenation of C14H29CH2 into C14H29CH3 species. The resulting CO is converted into CH4 according to CO → HCO → H2CO → CH2 → CH3 → CH4. The rate-limiting step is the formation of HCO. These calculation results clearly show that the C–O bond scission mechanism is favored over the C–C bond scission mechanism on Pt, whereas the opposite situation occurs on Ru; namely, this reaction is strongly metal dependent. The different mechanisms originate from different initial cleavage channels, in which Cα–H scission is preferred for 1-hexadecanol on Pt, whereas O–H scission is the dominant cleavage on Ru. Moreover, it was found that the selectivity and reactivity could be modified by modifying the experimental conditions such as H2 pressure. Confirming the mechanisms of C–C and C–O cleavage will aid the modification of experimental conditions to obtain high selectivity products. The present calculation results can be extended to other metals, such as Ni, bimetallic alloys, such as Ni/Pt(111), or other alkanol reaction systems.


 Please wait while we load your content...
Please wait while we load your content...