Elucidating and exploiting the chemistry of Keggin heteropolyacids in the methanol-to-DME conversion: enabling the bulk reaction thanks to operando Raman†
Abstract
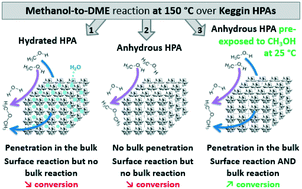
Operando Raman spectroscopy is used here to enlighten crucial and yet unconsidered aspects of the catalytic behavior of Keggin heteropolyacids (HPAs) in the gas phase dehydration of methanol to dimethylether (DME). On one hand, HPAs are since a long time claimed as being able to absorb methanol into their bulk, but on the other hand this feature is not yet really exploited when it comes to develop/use HPA-based catalysts for the methanol-to-DME process. Actually, the conditions in which the bulk is simultaneously accessible and catalytically active are not yet clearly reported. Clarifying this is precisely the aim of our work, for which we have used H3PW12O40 and H4SiW12O40. We precisely operando-follow the νs(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ot) band reflecting whether methanol penetrates or not in the HPAs' bulk. We show that, when the HPAs are used without having been beforehand completely dehydrated, methanol penetrates into their bulk. However, the conversion remains low (<5% at 150 °C) due to remaining crystallisation water reducing the availability of the active protons within the bulk, so limiting the activation of methanol. A complete dehydration pre-treatment is thus required if one wants to fully benefit of the bulk reactivity. However, we show that this benefit is obtained only if the pre-treatment is done adequately. The most intuitive pre-treatment, namely the HPAs' dehydration at about 300 °C and then cooling down to the reaction temperature 150 °C at which methanol is fed, is shown to be inefficient. This is due to the inaccessibility of the fully dehydrated bulk to methanol, so limiting the catalytic reaction to only the surface of the HPAs. At the opposite, a pre-treatment sequence consisting of dehydrating at 300 °C, then cooling down to 25 °C, exposing to methanol at this temperature, and then only heating to the reaction temperature, is shown to succeed combining penetration of methanol into the bulk and availability of the active protons. With such sequence, an unprecedented conversion of 40% is obtained thanks to a full exploitation of the HPAs' bulk. These results show that the HPAs' bulk can contribute significantly to convert methanol, provided that the catalysts are properly pre-treated.
Ot) band reflecting whether methanol penetrates or not in the HPAs' bulk. We show that, when the HPAs are used without having been beforehand completely dehydrated, methanol penetrates into their bulk. However, the conversion remains low (<5% at 150 °C) due to remaining crystallisation water reducing the availability of the active protons within the bulk, so limiting the activation of methanol. A complete dehydration pre-treatment is thus required if one wants to fully benefit of the bulk reactivity. However, we show that this benefit is obtained only if the pre-treatment is done adequately. The most intuitive pre-treatment, namely the HPAs' dehydration at about 300 °C and then cooling down to the reaction temperature 150 °C at which methanol is fed, is shown to be inefficient. This is due to the inaccessibility of the fully dehydrated bulk to methanol, so limiting the catalytic reaction to only the surface of the HPAs. At the opposite, a pre-treatment sequence consisting of dehydrating at 300 °C, then cooling down to 25 °C, exposing to methanol at this temperature, and then only heating to the reaction temperature, is shown to succeed combining penetration of methanol into the bulk and availability of the active protons. With such sequence, an unprecedented conversion of 40% is obtained thanks to a full exploitation of the HPAs' bulk. These results show that the HPAs' bulk can contribute significantly to convert methanol, provided that the catalysts are properly pre-treated.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles

 Please wait while we load your content...
Please wait while we load your content...