Mechanisms governing selective hydrogenation of acetylene over γ-Mo2N surfaces
Abstract
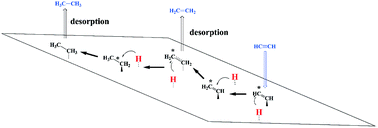
Selective hydrogenation of acetylene (ethyne), present in hydrocarbon feed into ethylene (ethene) plays critical importance in processing operations. Unsupported molybdenum-nitride (Mo2N) catalysts mediate surface hydrogenation of ethyne with a profound selectivity, similar to that observed over noble metals. However, the underlying mechanisms governing the H2–C2H2–Mo2N interaction leading to partial, rather than complete, hydrogenation remain largely unclear. In this contribution, we have found that molecular hydrogen adopts several physisorbed states prior to its dissociation, mainly on 3-fold hollow fcc (H1) and 4-fold hollow fcc (H3) sites over the (111) and (100) terminations of γ-Mo2N, respectively. Our results on the interaction of hydrogen with the γ-Mo2N surface concur very well with the analogous experimental findings of the dissociation occurring preferentially on the vacant nitrogen sites, the mobility of surface-adsorbed hydrogen atoms from weak to strong adsorption sites (associated with pathways of low and high activation energies for dissociation of adsorbed H2 on the catalyst surface) and the inhibition effect of pre-adsorbed oxygen on H2 dissociation. The constructed reaction mechanism, the estimated reaction rate constants, and the micro-kinetic modelling explain the selective hydrogenation of ethyne into ethene, rather than ethane. We demonstrate that the occurrence of selective hydrogenation rests on two aspects: distinctive energy profiles of the hydrogenation steps in partial versus full hydrogenation routes and thermodynamic selectivity entailing higher surface stability of adsorbed C2H2 in comparison to C2H4. Higher stabilities of the adsorbed open-shell CxHy species (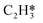 ,
, 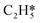 ) on the (100) surface, in comparison to those on the (111) surface, indicate increased tendency for oligomerisation to occur on the (100) surface. Thermo-kinetic parameters reported herein provide molecular-level understanding of the unique and highly selective hydrogenation capacity of the γ-Mo2N catalysts. Such knowledge is critically important in designing optimum operational conditions for practical processing operations.
) on the (100) surface, in comparison to those on the (111) surface, indicate increased tendency for oligomerisation to occur on the (100) surface. Thermo-kinetic parameters reported herein provide molecular-level understanding of the unique and highly selective hydrogenation capacity of the γ-Mo2N catalysts. Such knowledge is critically important in designing optimum operational conditions for practical processing operations.


 Please wait while we load your content...
Please wait while we load your content...