Benzodiazines: recent synthetic advances†
Abstract
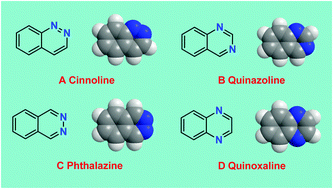
Benzodiazines (diazonaphthalenes with both nitrogens in the same ring) – cinnolines (1,2-benzodiazine), quinazolines (1,3-benzodiazine), phthalazines (2,3-benzodiazine) and quinoxalines (1,4-benzodiazine) – are important class of compounds with broad biological properties and wide application in pharmaceutical as well as agrochemical arenas. These diazaheterocycles are present in a wide variety of bioactive natural products as well as synthetic molecules that are good drug candidates constituting key structural units responsible for their pronounced therapeutic activities. Their rapidly growing uses and applications in medicinal and agrochemical arenas prompt the researchers for further studies on this important group of compounds. In this review, we hope to provide a brief overview of the important general methodologies and recent developments towards their synthesis and open the door for further progress in this area.


 Please wait while we load your content...
Please wait while we load your content...