Photoinduced dynamics in cycloparaphenylenes: planarization, electron–phonon coupling, localization and intra-ring migration of the electronic excitation†
Abstract
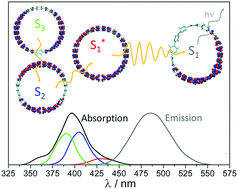
Cycloparaphenylenes represent the smallest possible fragments of armchair carbon nanotubes. Due to their cyclic and curved conjugation, these nanohoops own unique photophysical properties. Herein, the internal conversion processes of cycloparaphenylenes of sizes 9 through 16 are simulated using Non-Adiabatic Excited States Molecular Dynamics. In order to analyze effects of increased conformational disorder, simulations are done at both low temperature (10 K) and room temperature (300 K). We found the photoexcitation and subsequent electronic energy relaxation and redistribution lead to different structural and electronic signatures such as planarization of the chain, electron–phonon couplings, wavefunction localization, and intra-ring migration of excitons. During excited state dynamics on a picosecond time-scale, an electronic excitation becomes partially localized on a portion of the ring (about 3–5 phenyl rings), which is not a mere static contraction of the wavefunction. In a process of non-radiative relaxation involving non-adiabatic transitions, the latter exhibits significant dynamical mobility by sampling uniformly the entire molecular structure. Such randomized migration involving all phenyl rings, occurs in a wave-like fashion coupled to vibrational degrees of freedom. These results can be connected to unpolarized emission observed in single-molecule fluorescence experiments. Observed intra-ring energy transfer is subdued for lower temperatures and adiabatic dynamics involving low-energy photoexcitation to the first excited state. Overall our analysis provides a detailed description of photo excited dynamics in molecular systems with circular geometry, outlines size-dependent trends and connotes specific spectroscopic signatures appearing in time-resolved experimental probes.


 Please wait while we load your content...
Please wait while we load your content...