Urea hydrogen bond donor-mediated synthesis of high-index faceted platinum concave nanocubes grown on multi-walled carbon nanotubes and their enhanced electrocatalytic activity†
Abstract
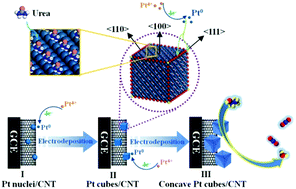
We have reported, for the first time, in situ growth of high-index {hk0} faceted concave Pt nanocubes on multi-walled carbon nanotubes (CNTs) via an electrochemical method in choline chloride–urea (ChCl–U) based deep eutectic solvents (DESs). Mechanistic studies indicate that a urea hydrogen bond donor (HBD) plays a key role in the formation of concave Pt nanocubes, in which the urea HBD preferentially adsorbs onto the {100} faces and blocks the growth of nanocrystals along the 〈100〉 axis. The as-prepared concave Pt nanocubes are characterized to be enclosed mainly with high-index {710}, {610} and {510} facets. It has been determined that the concave cubic Pt/CNT exhibits higher catalytic activity and stability than the flower-like Pt/CNT and commercial Pt/C catalysts, and this is ascribed to its high density of surface atomic steps and the synergistic effect between the CNT and Pt nanocubes.


 Please wait while we load your content...
Please wait while we load your content...