Catalytic CO2 reduction to valuable chemicals using NiFe-based nanoclusters: a first-principles theoretical evaluation†
Abstract
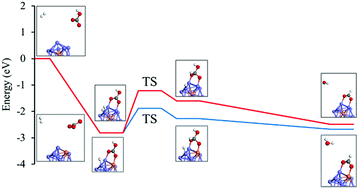
Converting CO2 into valuable chemicals and fuels is one of the most practical routes for reducing CO2 emissions while fossil fuels continue to dominate the energy sector. Noble-metal-free NiFe bimetal nanoparticles have shown good catalytic activity in CO2 conversion. Herein we theoretically evaluated the catalytic performance and possible mechanisms of NiFe-based nanoclusters for hydrogenating CO2 to form formic acid and CO through bicarbonate by using a periodic and self-consistent density functional theory (DFT) simulation. The theoretical results illustrated that NiFe nanoclusters could have good catalytic activity and selectivity for HCO3− reduction to formic acid and the possible pathway is that HCO3− preferred to react with adsorbed H atoms of H2 on NiFe alloy nanoclusters through the carbon atom site. Moreover, the NiFe alloy nanoclusters with the Fe atom exposed on the surface of the Ni cluster showed better performance with a lower energy barrier compared to that with Fe doped in the corner of the Ni cluster. However, the generation of CO from HCO3− reduction was shown to be neither thermodynamically nor kinetically favorable on NiFe alloy nanoclusters. Additionally, the simulation results also suggested that it was thermodynamically unfavorable for further hydrogenated reduction of formic acid to formaldehyde on NiFe alloy nanoclusters themselves as well as supported on graphene. In summary, a molecular-level insight of CO2 reduction to valuable products on NiFe nanoclusters is offered in this study, which may provide some useful information for guiding the design of NiFe-based catalytic materials for efficient CO2 conversion to useful fuels.


 Please wait while we load your content...
Please wait while we load your content...