Design of a catalyst through Fe doping of the boron cage B10H14 for CO2 hydrogenation and investigation of the catalytic character of iron hydride (Fe–H)†
Abstract
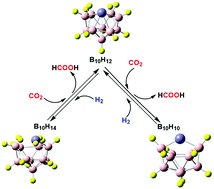
The innovative catalyst Fe@B10H14 is designed through Fe doping of the boron cage B10H14 and is employed to catalyze CO2 hydrogenation using a quantum mechanical method. First, the structure of the Fe@B10H14 complex is characterized through calculated 11B NMR chemical shifts and Raman spectra, and the interactions between Fe and the four H atoms of the opening in the cage are analyzed, which show that various iron hydride (Fe–H) characteristics exist. Subsequently, the potential of Fe@B10H14 as a catalyst for the hydrogenative reduction of CO2 in the gas phase is computationally evaluated. We find that an equivalent of Fe@B10H14 can consecutively reduce double CO2 to obtain the double product HCOOH through a two-step reduction, and Fe@B10H12 and Fe@B10H10 are successively obtained. The Fe presents single-atom character in the reduction of CO2, which is different from the common iron(II) catalyzed CO2 reduction. The calculated total free energy barrier of the first CO2 reduction is only 8.79 kcal mol−1, and that of the second CO2 reduction is 25.71 kcal mol−1. Every reduction reaction undergoes two key transition states TSC–H and TSO–H. Moreover, the transition state of the C–H bond formation TSC–H is the rate-determining step, where the interaction between πC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O* and the weak σFe–H bond plays an important role. Furthermore, the hydrogenations of Fe@B10H12 and Fe@B10H10 are investigated, which aim at determining the ability of Fe–H circulation in the Fe doped decaborane complex. We find that the hydrogenation of Fe@B10H10 undergoes a one-step H2-adsorbed transition state TSH-adsorb with an energy barrier of 6.42 kcal mol−1 from Fe@B10H12. Comparing with the hydrogenation of Fe@B10H10, it is slightly more difficult for the hydrogenation of Fe@B10H12, where the rate-determining step is the H2-cleaved transition state TS2H–H with an energy barrier of 17.38 kcal mol−1.
O* and the weak σFe–H bond plays an important role. Furthermore, the hydrogenations of Fe@B10H12 and Fe@B10H10 are investigated, which aim at determining the ability of Fe–H circulation in the Fe doped decaborane complex. We find that the hydrogenation of Fe@B10H10 undergoes a one-step H2-adsorbed transition state TSH-adsorb with an energy barrier of 6.42 kcal mol−1 from Fe@B10H12. Comparing with the hydrogenation of Fe@B10H10, it is slightly more difficult for the hydrogenation of Fe@B10H12, where the rate-determining step is the H2-cleaved transition state TS2H–H with an energy barrier of 17.38 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...