Quantification of cation–anion interactions in crystalline monopotassium and monosodium glutamate salts†
Abstract
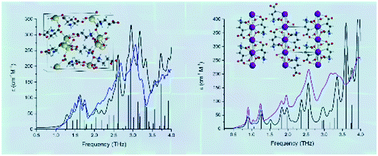
Crystalline salt compounds composed of metal cations and organic anions are becoming increasingly popular in a number of fields, including the pharmaceutical and food industries, where such formulations can lead to increased product solubility. The origins of these effects are often in the interactions between the individual components in the crystals, and understanding these forces is paramount for the design and utilisation of such materials. Monosodium glutamate monohydrate and monopotassium glutamate monohydrate are two solids that form significantly different structures with correspondingly dissimilar dynamics, while their chemistry only differs in cation identity. Crystals of each were characterised experimentally with single-crystal X-ray diffraction and terahertz time-domain spectroscopy and theoretically using solid-state density functional theory simulations, in order to explain the observed differences in their bulk properties. Specifically, crystal orbital overlap and Hamiltonian population analyses were performed to examine the role that the individual interactions between the cation and anion played in the solid-state structures and the overall energetic profiles of these materials.


 Please wait while we load your content...
Please wait while we load your content...