Substitution pattern on anthrol carbaldehydes: excited state intramolecular proton transfer (ESIPT) with a lack of phototautomer fluorescence†
Abstract
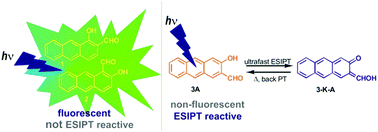
Photophysical properties and excited state intramolecular proton transfer (ESIPT) reactivity for anthrol carbaldehydes 1–5 have been investigated computationally and experimentally by steady-state and time-resolved fluorescence and laser flash photolysis (LFP). 1,2-Disubstituted anthrol carbaldehydes 1 and 2 are not ESIPT reactive, contrary to naphthol analogues. The main deactivation channels from S1 for 1 and 2 are fluorescence (ΦF = 0.1–0.2) and intersystem crossing (ISC) to almost isoenergetic T2 states. The triplet states from 1 and 2 were detected by LFP (in N2-purged CH3CN, τ = 15 ± 2 μs for 1, and τ = 5.5 ± 0.1 μs for 2). In contrast, 2,3-disubstituted anthrols 3–5 undergo efficient barrierless ultrafast ESIPT. However, the typical dual emission from locally excited states and ESIPT tautomers were not observed since ESIPT proceeds via a conical intersection with S0 delivering the keto-tautomer in the hot ground state. Therefore, anthrols 3–5 are about ten times less fluorescent compared to 1 and 2, and the emission for 3–5 originates from less-populated conformers that cannot undergo ESIPT. Keto-tautomers for 3–5 were detected in CH3CN by LFP (λmax = 370 nm, τ = 30–40 ns). The difference in ESIPT reactivity for 1–3 was fully disclosed by calculations at ADC(2)/aug-cc-pVDZ level of theory, and particularly, by calculation of charge redistribution upon excitation to S1. Only 2,3-disubstituted anthrols exhibit polarization in S1 that increases the electron density on the carbonyl and decreases this density on the phenolic OH, setting the stage for ultrafast ESIPT.


 Please wait while we load your content...
Please wait while we load your content...