A mechanistic study of the Knoevenagel condensation reaction: new insights into the influence of acid and base properties of mixed metal oxide catalysts on the catalytic activity†
Abstract
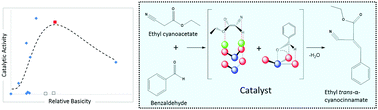
A Knoevenagel condensation reaction between benzaldehyde and ethyl cyanoacetate was performed in the liquid phase under mild and solventless conditions using a series of catalysts modified by impregnating magnesium and barium cations on different supports (SiO2, ZnO, γ-Al2O3 and Fe2O3). The highest reaction rates and yields (after 6 hours) were observed using ZnO which possessed the highest concentration of acidic and basic sites, as determined by TPD-MS. In situ FTIR experiments show that the adsorption of ethyl cyanoacetate on ZnO results in an increase of hydroxyl intermediate species on the ZnO surface. There is evidence to suggest that the reaction proceeds via an autocatalytic pathway, as an induction period was observed before the observed catalytic activity. It was also found that both surface acidic and basic sites are necessary to obtain high rates of the catalytic reaction. By comparing the catalytic activity with the catalyst surface basicity, we are able to determine the optimum proportion of acid and base sites necessary to obtain the highest reaction rates.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...