Investigation into the energy storage behaviour of layered α-V2O5 as a pseudo-capacitive electrode using operando Raman spectroscopy and a quartz crystal microbalance
Abstract
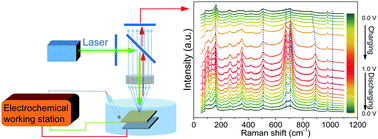
α-V2O5 nanowires with a layered structure have been fabricated through a two-step procedure. When measured as a pseudo-capacitive electrode with a three-electrode configuration in 1 M Na2SO4 aqueous solutions, α-V2O5 exhibits ideal capacitive characteristics with a specific capacitance of ∼238 F g−1 at a high current of 2 A g−1, but poor cycling stability with a continuous drop in the first 2000 cycles before it is maintained. To find possible solutions towards this problem, the energy storage behavior of the α-V2O5 electrode has been carefully investigated. In situ Raman analysis suggests that it is electrolytic hydrated cations [Na(H2O)n]+ rather than anions (SO42−) that are involved in the energy storage process through reversible adsorption/desorption on the surface or intercalation/deintercalation at the interlayer of the (001) planes accompanied by interlayer spacing expansion/contraction. Moreover, the electrochemical quartz crystal microbalance results indicate that, besides a reversible mass change, there is a continuous mass loss that may originate from slow dissolution of V2O5, which should bear the main responsibility for the poor stability (initial dramatic drop). Hence, how to inhibit dissolution, such as by coating or adding additives in the electrolyte, is found to be the key approach to improve the stability of V2O5 based electrodes.


 Please wait while we load your content...
Please wait while we load your content...