Nonexponential kinetics of ion pair dissociation in electrofreezing water†
Abstract
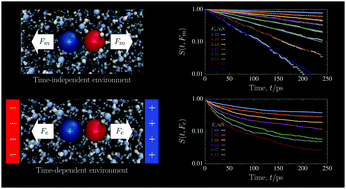
Temporally- or spatially-heterogeneous environments can participate in many kinetic processes, from chemical reactions and self-assembly to the forced dissociation of biomolecules. Here, we simulate the molecular dynamics of a model ion pair forced to dissociate in an explicit, aqueous solution. Triggering dissociation with an external electric field causes the surrounding water to electrofreeze and the ion pair population to decay nonexponentially. To further probe the role of the aqueous environment in the kinetics, we also simulate dissociation events under a purely mechanical force on the ion pair. In this case, regardless of whether the surrounding water is a liquid or already electrofrozen, the ion pair population decays exponentially with a well-defined rate constant that is specific to the medium and applied force. These simulation data, and the rate parameters we extract, suggest the disordered kinetics in an electrofreezing medium are a result of the comparable time scales of two concurrent processes, electrofreezing and dissociation.


 Please wait while we load your content...
Please wait while we load your content...