Ultrafast flavin photoreduction in an oxidized animal (6-4) photolyase through an unconventional tryptophan tetrad†
Abstract
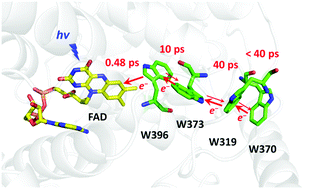
Photolyases are flavoenzymes repairing UV-induced lesions in DNA, which may be activated by a photoreduction of their FAD cofactor. In most photolyases, this photoreduction proceeds by electron transfer along a chain of three tryptophan (Trp) residues, connecting the flavin to the protein surface. Much less studied, animal (6-4) photolyases (repairing pyrimidine–pyrimidone (6-4) photoproducts) are particularly interesting as they were recently shown to have a longer electron transfer chain, counting four Trp residues. Using femtosecond polarized transient absorption spectroscopy, we performed a detailed analysis of the photoactivation reaction in the (6-4) photolyase of Xenopus laevis with oxidized FAD. We showed that the excited flavin is very quickly reduced (∼0.5 ps) by a nearby tryptophan residue, yielding FAD˙− and WH˙+ radicals. Subsequent kinetic steps in the picosecond regime were assigned to the migration of the positive charge along the Trp tetrad, in competition with charge recombination. We propose that the positive charge is actually delocalized over various Trp residues during most of the dynamics and that charge recombination essentially occurs through the proximal tryptophanyl radical. Oxidation of the fourth tryptophan is thought to be reached about as fast as that of the third one (∼40 ps), based on a comparison with a mutant protein lacking the distal Trp, implying ultrafast electron transfer between these two residues. This unusual mechanism sheds light on the rich diversity of electron transfer pathways found in various photolyases, and evolution-related cryptochromes alike.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...