Electrolyte containing lithium cation in squaraine-sensitized solar cells: interactions and consequences for performance and charge transfer dynamics†
Abstract
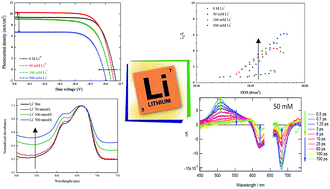
By optimizing the lithium concentration in an electrolyte to 50 mmol L−1 and the dye-to-chenodeoxycholic acid ratio in a VG1-based dye solution, we achieved 4.7% power conversion efficiency under standard AM 1.5G conditions. In addition to this performance, we herein discuss the role played by lithium in the electrolyte and its interplay in the charge transfer processes from ms to fs dynamics. Based on electrochemical impedance spectroscopy, photoluminescence and pump–probe transient absorption spectroscopy, we conclude that although lithium increases the electron diffusion length, this has no satisfactory impact on electron injection and even slows dye regeneration. This study provides evidence that lithium is not only specifically adsorbed on the surface of TiO2 but prompts a molecular reorganization of the self-assembled dye monolayer, forming harmful H-aggregates.


 Please wait while we load your content...
Please wait while we load your content...