Isolated complexes of the amino acid arginine with polyether and polyamine macrocycles, the role of proton transfer
Abstract
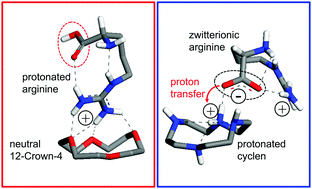
The distinct basicity of the guanidinium side-group of arginine (Arg) sustains specific interactions involved in essential biochemical processes. The sensing of arginine is therefore key in modern biotechnology and bioanalysis. In this context, the development of molecular receptors based on crown ether building blocks has demonstrated great potential. We investigate the complexes formed by arginine with two benchmark macrocycles, 12-crown-4 (1,4,7,10-tetraoxacyclododecane) and its N-substituted analog cyclen (1,4,7,10-tetraazacyclododecane). Isolated complexes with a net charge +1 are characterized with infrared action vibrational spectroscopy and quantum mechanical computations in order to determine the most stable coordination arrangements and to elucidate the location of the protons involved. Remarkably, although arginine retains a net positive charge in its complex with 12-crown-4, it becomes zwitterionic in the cyclen complex. In this latter case, the guanidinium group remains protonated while a proton transfer from the carboxylic group occurs, leading to a charged −NH2+ moiety in cyclen. Natural bond orbital analysis is employed to characterize the intermolecular H-bonds responsible for the stability of both complexes. Protonated arginine interacts with 12-crown-4 through the guanidinium side group, in a conformation that resembles the one expected for crown–Arg binding in peptidic chains. In contrast, the cyclen complex involves the coordination of the carboxylate anionic group with a N–H bond of the protonated amine group cyclen, and plausible but less relevant interactions with the guanidinium group.


 Please wait while we load your content...
Please wait while we load your content...