Theoretical study on the reaction mechanism of hydrogenation of furfural to furfuryl alcohol on Lewis acidic BEA zeolites: effects of defect structure and tetravalent metals substitution†
Abstract
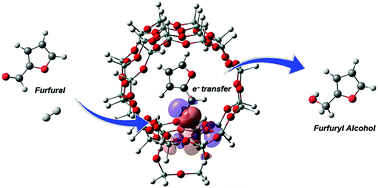
Furfural acquired from agricultural sources is receiving extensive attention in the petrochemical industry as it offers an alternative route to generate more valuable hydrocarbon compounds. Herein, we investigate the furfural hydrogenation to furfuryl alcohol catalyzed by Lewis acidic BEA zeolites at the molecular level by means of the M06-L density functional theory. The mechanistic pictures in the catalytic procedure are revealed. The possible reaction pathways are considered to proceed via either concerted or stepwise mechanisms. With the contribution of zeolite oxygen bridging for the H–H splitting, the rate determining step activation barrier for the stepwise mechanism is 14.7 kcal mol−1 lower than that for the concerted mechanism. The stepwise reaction therefore seems to be favored compared to the concerted one. The catalytic effect of the defect zeolite framework on the stepwise mechanism is also investigated. The activation energy for the stepwise rate-determining step over this site is significantly lower than the corresponding step over the perfect one by 14.1 kcal mol−1. Finally, the catalytic activity of tetravalent metal centers (Sn, Ge, Zr and Hf) substituted in BEA is also preliminarily compared and it is found to follow the order of Hf > Zr > Sn > Ge based on activation energies and the reaction rate. The difference in the activation energy can be traced back to the difference in the charge transfer from the catalytic site to the adsorbed molecules.


 Please wait while we load your content...
Please wait while we load your content...