Structural features of monohydrated 2-(4-fluorophenyl)ethylamine: a combined spectroscopic and computational study†
Abstract
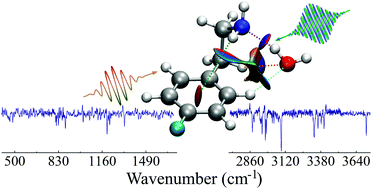
A jet-cooled singly hydrated 2-(4-fluorophenyl)ethylamine (4-FPEA–H2O) cluster has been studied by ionization-loss stimulated Raman spectroscopy of the 4-FPEA photofragment and density functional calculations of the parent. Comparison of the measured spectrum of the photofragment to computed scaled harmonic Raman spectra of different conformers of the 4-FPEA–H2O cluster, at the M06-2X/6-311++G(d,p) level of theory, allowed determination of the calculated spectrum that best fits the experimental one. The correlation between them was further supported by the stability of the cluster, as revealed from the calculated energies of the fully optimized geometries of the possible different clusters in the ground electronic state. The corresponding structure consists of a water molecule, which is hydrogen-bonded to the nitrogen lone pair of the folded ethylamino side chain in the most stable gauche conformer of 4-FPEA. The presence of the hydrogen bond and other bonding and non-bonding interactions was also tested by atoms in molecules and noncovalent interaction analyses. The former approach showed no critical points in electron density, while the latter revealed regional topologies of reduced density gradients, indicating the formation of this hydrogen-bond and other attractive and repulsive interactions. The monohydration of 4-FPEA provides an insight into the intra- and inter-molecular interactions that play a role in stabilizing the cluster.


 Please wait while we load your content...
Please wait while we load your content...