Two coexisting liquid phases in switchable ionic liquids†
Abstract
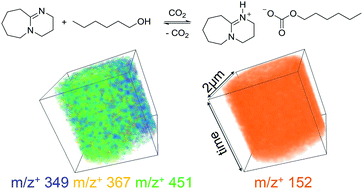
Switchable ionic liquids (SWILs) derived from organic bases and alcohols are attractive due to their applications in gas capture, separations, and nanomaterial synthesis. However, their exact solvent structure still remains a mystery. We present the first chemical mapping of a SWIL solvent structure using in situ time-of-flight secondary ion mass spectrometry. In situ chemical mapping discovers two coexisting liquid phases and molecular structures vastly different from conventional ionic liquids. SWIL chemical speciation is found to be more complex than the known stoichiometry. Dimers and ionic clusters have been identified in SIMS spectra; and confirmed to be the chemical species differentiating from non-ionic liquids via spectral principal component analysis. Our unique in situ molecular imaging has advanced the understanding of SWIL chemistry and how this “heterogeneous” liquid structure may impact SWILs’ physical and thermodynamic properties and associated applications.


 Please wait while we load your content...
Please wait while we load your content...