The effects of ice on methane hydrate nucleation: a microcanonical molecular dynamics study†
Abstract
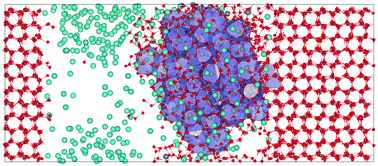
Although ice powders are widely used in gas hydrate formation experiments, the effects of ice on hydrate nucleation and what happens in the quasi-liquid layer of ice are still not well understood. Here, we used high-precision constant energy molecular dynamics simulations to study methane hydrate nucleation from vapor–liquid mixtures exposed to the basal, prismatic, and secondary prismatic planes of hexagonal ice (ice Ih). Although no significant difference is observed in hydrate nucleation processes for these different crystal planes, it is found, more interestingly, that methane hydrate can nucleate either on the ice surface heterogeneously or in the bulk solution phase homogeneously. Several factors are mentioned to be able to promote the heterogeneous nucleation of hydrates, including the adsorption of methane molecules at the solid–liquid interface, hydrogen bonding between hydrate cages and the ice structure, the stronger ability of ice to transfer heat than that of the aqueous solution, and the higher occurrence probability of hydrate cages in the vicinity of the ice surface than in the bulk solution. Meanwhile, however, the other factors including the hydrophilicity of ice and the ice lattice mismatch with clathrate hydrates can inhibit heterogeneous nucleation on the ice surface and virtually promote homogeneous nucleation in the bulk solution. Certainly, the efficiency of ice as a promoter and as an inhibitor for heterogeneous nucleation is different. We estimate that the former is larger than the latter under the working conditions. Additionally, utilizing the benefit of ice to absorb heat, the NVE simulation of hydrate formation with ice can mimic the phenomenon of ice shrinking during the heterogeneous nucleation of hydrates and lower the overly large temperature increase during homogeneous nucleation. These results are helpful in understanding the nucleation mechanism of methane hydrate in the presence of ice.


 Please wait while we load your content...
Please wait while we load your content...