Long-range Li ion diffusion in NASICON-type Li1.5Al0.5Ge1.5(PO4)3 (LAGP) studied by 7Li pulsed-gradient spin-echo NMR†
Abstract
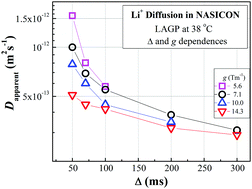
The long-range Li ion diffusion in Li1.5Al0.5Ge1.5(PO4)3 (LAGP), a NASICON-type glass ceramic conductor with high ionic conductivity, was studied using pulsed-gradient spin-echo (PGSE) 7Li NMR. LAGP is stable in air and water, and can be used for all-solid Li batteries as well as next generation Li-air batteries. The Li ion conduction mechanisms in the micrometer space are important for the design of Li batteries and development of new materials. Our previous studies on sulfide-based and garnet-type solid conductors showed that uniform Li+ ion diffusion is hampered by narrow pathways surrounded by stationary anions. The Li ions are engaged in parameter-dependent diffusion with the parameters being observation time (Δ) and pulsed-field gradient (PFG) strength (g); this is completely different from the Li diffusion in solution and polymer electrolytes, and also from molecular diffusion in neutral porous media. In this study, we observed apparent diffusion constant (Dapparent) values for the LAGP, that were almost continuously distributed within a limited range at a certain Δ. At very long observation times (above 300 ms) and large g (∼13 Tm−1), an equilibrated diffusion constant (close to the tracer diffusion constant) could be observed. The apparent activation energy of Li ion diffusion was about 16 kJ mol−1, which was smaller than the activation energy for the ionic conductivity. The temperature-dependent carrier ion numbers were estimated.


 Please wait while we load your content...
Please wait while we load your content...