The influence of an adsorbate and edge covalent bonds on topological zero modes in few-layer nanographenes
Abstract
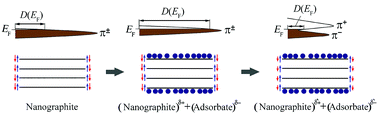
The existence of zero-energy edge π-electronic states (zero modes) in turbostratic few-layer nanographenes (nanographites) has been established and the sensitivity of their characteristics to the presence of adsorbed chlorine molecules and covalent bonds of halogen with dangling edge carbon orbitals has been studied using a combination of wide- and small-angle X-ray diffraction, Raman spectroscopy, XPS, EPR and magnetic susceptibility experiments. The reversible change in density of the edge π-electronic states found under the influence of an adsorbate has been explained by their spin-splitting initiated with the transfer of a small part of the electron density from the nanographites to the adsorbate. The change of the sign of the temperature coefficient of the current carrier spin relaxation rate caused by the adsorbate has also been accounted for in the framework of this model as a consequence of interaction of mobile spins with edge spin-split (magnetically ordered) states. Our investigations have shown the preservation of π-electronic states with zero energy on chlorine saturation of free (dangling) σ-orbitals of edge carbon atoms.


 Please wait while we load your content...
Please wait while we load your content...