Structural insights into the multinuclear speciation of tetravalent cerium in the tri-n-butyl phosphate–n-dodecane solvent extraction system†
Abstract
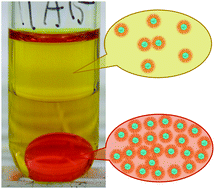
X-ray and electrochemical studies of organic phases obtained by the extraction of tetravalent cerium, Ce(IV), from aqueous nitric acid (3 M) with tri-n-butyl phosphate (TBP) in n-dodecane reveal a tetranuclear Ce(IV) structural motif. This finding is consistent with the results of previous liquid–liquid extraction (LLE) studies that implicate the aggregation of (Ce–O–Ce)6+ dimers into multinuclear Ce(IV)·TBP solvates. The organic solution structures elaborated here for the Ce(IV)–HNO3–20% TBP-n-C12H26 system are correlated with multiscale phenomena—from the atomic level of the cerium coordination environment to the supramolecular scale of solute aggregates—in the organic phases, which are of relevance to the PUREX (Plutonium Uranium Reduction EXtraction) process. The combination of XANES, EXAFS, and SAXS results indicate the presence of tetranuclear cerium(IV)–oxo core structures in each of the organic phases investigated. In addition to the use of X-ray spectroscopy and scattering for direct metrical details about the organic phase solute speciation, three-phase-electrode differential pulse voltammetry (DPV) of the third phase reveals a wave attributable to Ce(IV) reduction. The electrode potential is consistent with values for the reduction of Ce(IV) in (Ce–O–Ce)6+ dimers in aqueous electrolytes. The Ce(IV) coordination chemistry of the organic solvates is independent of the bulk phenomenon of phase splitting, namely third phase formation. The local, molecular environment of Ce in the organic phase before splitting is identical to those in the two organic phases (the dense third phase and the light phase) after splitting. SAXS data are consistent with the formation of small spherical reverse micelles with core diameters (approx. 6 Å) that can accommodate a tetranuclear Ce(IV) oxo-cluster solvate of TBP. Sticky sphere modeling of the SAXS data for the organic phases with low cerium concentrations (<0.14 M) is consistent with the presence of randomly- and homogenously-dispersed micelles in combination with short-range percolated, associated micelles. At high cerium concentrations (approx. 1.5 M) in the third phase, the SAXS modeling is consistent with correlated, long-range percolated micellar aggregates. The presence of strong inter-micellar interactions (−3 to −5kBT) in all organic phases of the Ce(IV)–HNO3–TBP–n-C12H26 LLE system suggests that the phenomena of phase splitting and third phase inversion are due to liquid precipitation that is dependent solely on the concentration of the tetranuclear Ce solvate.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...