A comparative VUV absorption mass-spectroscopy study on protonated peptides of different size
Abstract
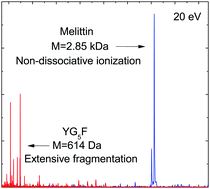
The ionization of gas-phase protonated peptides and proteins can induce molecular responses ranging from purely non-dissociative ionization to extensive multifragmentation of the system. In the case of soft X-ray photoionization, a monotonic transition between both regimes occurs in the mass range between 0.5 and 10 kDa. Despite the localized nature of the photoabsorption, excitation energy equilibrates before fragmentation sets in and the transition reflects the increase of the heat capacity with protein size. Here, we have investigated the influence of peptide size on vacuum ultraviolet (VUV) photoionization of protonated proteins, where photoexcitation and ionization are limited to valence electrons rather than inner shell electrons and the photoexcitation contribution is markedly lower. Gas phase protonated peptides with masses ranging from 0.6–2.8 kDa were trapped in a radiofrequency ion trap and exposed to synchrotron radiation. Time of flight mass spectrometry was employed for the investigation of the photoionization and photofragmentation processes. The relationship between peptide fragmentation and peptide size exhibits a similar trend as observed for soft X-ray absorption. Due to the lower excitation energies involved, however, dissociation is already quenched at smaller masses and peptide amino acid compositions, protonation states and ionization potentials lead to deviations from the general trend.


 Please wait while we load your content...
Please wait while we load your content...