Complexation induced aggregation and deaggregation of acridine orange with sulfobutylether-β-cyclodextrin
Abstract
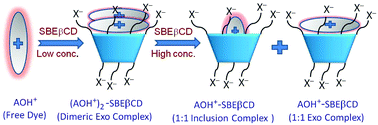
The present study reports a contrasting interaction behaviour of a biologically important dye, acridine orange (AOH+), with a highly water soluble anionic host, based on a β-cyclodextrin (βCD) scaffold, i.e. sulfobutylether-β-cyclodextrin (SBEβCD), in comparison to native βCD. AOH+ shows striking modulation in its photophysical properties, representing sequential changes in the modes of interaction with increasing SBEβCD concentration. At lower SBEβCD concentrations, AOH+ preferentially binds in dimeric forms at the negatively charged SBEβCD portals, leading to strong fluorescence quenching. At higher SBEβCD concentrations, the dimeric dyes convert to monomeric forms and subsequently undergo both inclusion and exo complex formation with 1 : 1 stoichiometry, resulting in a large fluorescence enhancement. The intriguing observation of sequential fluorescence switch off and switch on for an AOH+–SBEβCD system is clearly facilitated by the presence of butylether chains with SO3− end groups at the portals of SBEβCD, providing an additional ion–ion interaction and much enhanced hydrophobic interaction for cationic AOH+ compared to the native βCD host. To the best of our knowledge, such fluorescence off/on switching through multistep host–guest binding has not been reported so far in the literature. The present study not only provides a detailed insight into the unique binding interactions of AOH+ with the SBEβCD host, but the findings of this study are also expected to be useful in designing supramolecular based drug formulations, drug delivery systems, sensors, and so on.


 Please wait while we load your content...
Please wait while we load your content...