Energetic and spectroscopic properties of the low-lying C7H2 isomers: a high-level ab initio perspective†
Abstract
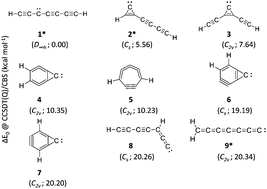
We use high-level ab initio CCSD(T) and CCSDT(Q) methods to investigate the energetic and spectroscopic properties of nine low-lying isomers of C7H2, which lie within 1 eV. Among these, heptatriynylidene (1), 1-(buta-1,3-diynyl)cyclopropenylidene (2) and heptahexaenylidene (9) have been detected experimentally. The other six isomers, 1,2-(diethynyl)cyclopropenylidene (3), bicyclo[4.1.0]hepta-1,2,4,5-tetraene-7-ylidene (4), cyclohepta-1,2,3,4-tetraen-6-yne (5), bicyclo[4.1.0]hepta-4,6-diene-2-yne-7-ylidene (6), bicyclo[4.1.0]hepta-1,5-diene-3-yne-7-ylidene (7) and 1-(buta-1,3-diynyl)propadienylidene (8), remain hypothetical to date. Except for 1, all of the isomers are associated with a non-zero dipole moment (μ ≠ 0). Although Fourier-transform microwave spectroscopy had detected 2 and 9, our study reveals that six hypothetical isomers (3–8) are thermodynamically sandwiched between the experimentally known and astronomically relevant isomers 2 and 9. The structural parameters, dipole moments, rotational and centrifugal distortion constants, harmonic vibrational frequencies, and infra-red intensities presented here may be useful for the laboratory detection of these previously unidentified isomers (3–8) and also all others (2–9) in astronomical sources.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...