B–H⋯π: a nonclassical hydrogen bond or dispersion contact?†
Abstract
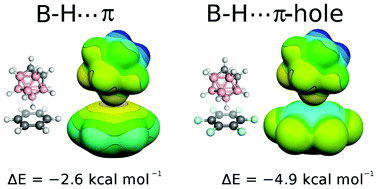
Close B–H⋯π contacts have recently been observed in crystallographic structures of Ir–dithiolene–phosphine complexes containing boron hydride cluster. This finding was interpreted using quantum chemical calculations as a new type of electrostatically driven nonclassical hydrogen bonding. However, such an explanation contradicts the wealth of evidence for unique noncovalent interactions of boron hydrides. Moreover, care must be exercised when computational methods are used to interpret new phenomena. Therefore, here, we cautiously examine the B–H⋯π interaction by means of advanced quantum chemistry and disprove the claimed attractive electrostatic nature and rather define it as a nonspecific dispersion-driven contact. In summary, we present evidence that the crystallographically observed B–H⋯π contacts do not constitute a novel type of hydrogen bonding of boron hydride clusters.


 Please wait while we load your content...
Please wait while we load your content...