Long-lived excited states in metal clusters†
Abstract
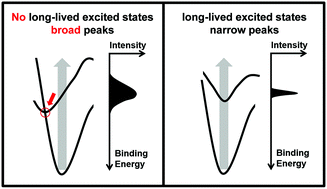
Bare metal clusters have properties that make them interesting for applications in photochemistry and photovoltaics. Long-lived excited states are a prerequisite for such applications, because in them the energy of the photon can be stored. Clusters have a low density of states and long-lived excited states should therefore occur frequently. However, in fact, such states are a rarity, as indicated by time-resolved photoelectron data of mass-selected cluster anions. And there is another puzzling observation: only clusters with narrow peaks in their photoelectron spectra exhibit long-lived excited states. Both findings can be explained if internal conversion, i.e. the conversion of electronic excitation energy into vibrational excitations, is the major relaxation mechanism in clusters. It becomes more likely, if a change of the electronic configuration results in a large geometry change, which is probably the case for most clusters. Only clusters with a weak coupling between geometric and electronic structure may have long-lived excited states and narrow peaks.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...