Mechanistic insights into dioxygen activation, oxygen atom exchange and substrate epoxidation by AsqJ dioxygenase from quantum mechanical/molecular mechanical calculations†
Abstract
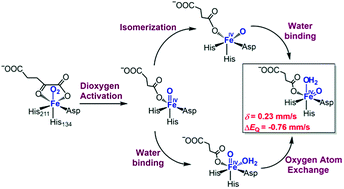
Herein, we use in-protein quantum mechanical/molecular mechanical (QM/MM) calculations to elucidate the mechanism of dioxygen activation, oxygen atom exchange and substrate epoxidation processes by AsqJ, an FeII/α-ketoglutarate-dependent dioxygenase (α-KGD) using a 2-His-1-Asp facial triad. Our results demonstrated that the whole reaction proceeds through a quintet surface. The dioxygen activation by AsqJ leads to a quintet penta-coordinated FeIV–oxo species, which has a square pyramidal geometry with the oxo group trans to His134. This penta-coordinated FeIV–oxo species is not the reactive one in the substrate epoxidation reaction since its oxo group is pointing away from the target C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Instead, it can undergo the oxo group isomerization followed by water binding or the water binding followed by oxygen atom exchange to form the reactive hexa-coordinated FeIV–oxo species with the oxo group trans to His211. The calculated parameters of Mössbauer spectra for this hexa-coordinated FeIV–oxo intermediate are in excellent agreement with the experimental values, suggesting that it is most likely the experimentally trapped species. The calculated energetics indicated that the rate-limiting step is the substrate C
C bond. Instead, it can undergo the oxo group isomerization followed by water binding or the water binding followed by oxygen atom exchange to form the reactive hexa-coordinated FeIV–oxo species with the oxo group trans to His211. The calculated parameters of Mössbauer spectra for this hexa-coordinated FeIV–oxo intermediate are in excellent agreement with the experimental values, suggesting that it is most likely the experimentally trapped species. The calculated energetics indicated that the rate-limiting step is the substrate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond activation. This work improves our understanding of the dioxygen activation by α-KGD and provides important structural information about the reactive FeIV–oxo species.
C bond activation. This work improves our understanding of the dioxygen activation by α-KGD and provides important structural information about the reactive FeIV–oxo species.


 Please wait while we load your content...
Please wait while we load your content...