Theoretical tuning of the singlet–triplet energy gap to achieve efficient long-wavelength thermally activated delayed fluorescence emitters: the impact of substituents†
Abstract
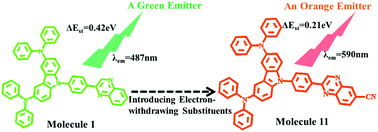
Great progress has been made in developing highly efficient thermally activated delayed fluorescent (TADF) materials. However, developing highly efficient long-wavelength TADF emitters is still a challenge because a small energy gap (ΔEST) between the lowest singlet (S1) and triplet excited states (T1) and a relatively high fluorescence rate are difficult to achieve simultaneously in one molecule. Here, eleven donor–acceptor (D–A) type molecules using N3,N3,N6,N6-tetraphenyl-9H-carbazole-3,6-diamine (named DAC-II) as the electron donor and the 2-phenyl-quinoxaline-based electron acceptor are designed via introducing different electron-donating and electron-withdrawing groups into the acceptor and changing the connection position between the donor (D) and the acceptor (A). Quantum chemical calculations indicated that introducing the electron-donating groups (–OCH3, –CH3) into the phenyl ring of the acceptor, molecules 2 and 3, cannot change the emission property of molecule 1, thus molecules 2 and 3 could also be used as green TADF emitters like molecule 1. Introducing an electron-withdrawing unit (–CF3) into molecule 1, molecule 4, reduces the ΔEST value to 0.10 eV, while the radiative decay rate (kVE) is also reduced correspondingly. Changing the connection position between D and A, molecules 5 to 8, cannot reduce the ΔEST value and lowers the kVE value compared with molecules 1 to 4. However, introducing electron-withdrawing groups (–2F, –4F and –CN) into the quinoxaline moiety, molecules 9 to 11, contributes to both small ΔEST and large kVE for the emission process. The values of ΔEST of molecules 9 to 11 are in the range of 0.21 to 0.30 eV, and the maximum emission wavelengths of molecules 10 and 11 are 576 and 590 nm, respectively, which are promising to be used as efficient yellow and orange TADF emitters in organic light-emitting diodes.


 Please wait while we load your content...
Please wait while we load your content...