Cu supported on mesoporous ceria: water gas shift activity at low Cu loadings through metal–support interactions†
Abstract
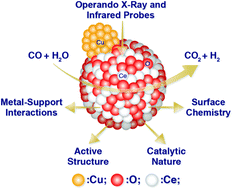
We have synthesized and tested a highly active Cu doped mesoporous CeO2 catalyst system for the low temperature water-gas shift (WGS) reaction. While typical oxide-supported copper WGS catalysts are characterized by high copper loadings (30–40%), the morphological properties of the mesoporous CeO2 material enable high catalytic activity at copper loadings as low as 1%. Operando X-ray diffraction, in situ X-ray absorption near-edge structure spectroscopy (XANES), and operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) methods were used to probe the interactions between the metal and mesoporous oxide components under reaction conditions. Copper was observed to undergo reduction from oxide to metal under WGS conditions at 150 °C, while the CeO2 lattice was observed to expand upon heating, indicating Ce3+ formation correlated with CO2 production. The active state of the catalysts was confirmed by in situ XANES to contain Cu0 and partially reduced CeO2. DRIFTS analysis revealed carboxyl species bound to copper during reduction, as well as formate and carbonate surface species on ceria. Lower concentrations of copper were observed to foster enhanced metal–support interactions.


 Please wait while we load your content...
Please wait while we load your content...