Antiradical activity of catecholamines and metabolites of dopamine: theoretical and experimental study†
Abstract
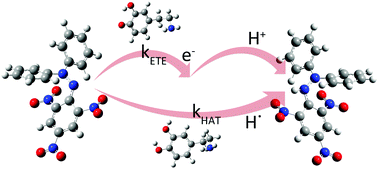
The importance of molecules with antiradical potency that are produced in the human body has significantly increased. Among others, neurotransmitters and their metabolites act as the first line of defense against oxidative stress in the peripheral endocrine and the central nervous systems. Dopamine (DO), epinephrine (EP), norepinephrine (NE), L-DOPA, catechol, and three metabolites of dopamine (3-methoxytyramine (3-MT), homovanillic acid (HO), and 3,4-dihydrophenylacetic acid (DOPAC)) were investigated for their antiradical potency via computational methods and DPPH assay. Density functional theory calculations were used to determine the most probable reaction mechanism based on the thermodynamic parameters. These results suggested that hydrogen atom transfer (HAT)/proton-coupled electron transfer (PCET) and sequential proton loss electron transfer (SPLET) mechanisms are preferable in polar solvents. Several techniques were employed to differentiate between HAT and PCET mechanisms via examination of the transition state structures. Kinetic studies of HAT/PCET and electron transfer (ET) reactions, the second step of SPLET, have proven that ET is much faster for an order of 105–106. Based on this, it was concluded that SPLET was the dominant mechanism for the antiradical activity towards DPPH radicals in polar solvents. The findings suggest that all the investigated molecules can be classified as excellent antiradical scavengers, except for 3-MT and homovanillic acid.


 Please wait while we load your content...
Please wait while we load your content...