Study of antiradical mechanisms with dihydroxybenzenes using reaction force and reaction electronic flux†
Abstract
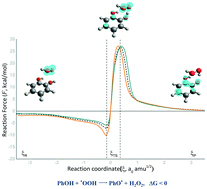
Phenolic compounds represent an important category of antioxidants because they help inhibit the oxidation process of organic compounds, while also acting as antiradicals in many biological processes. In this work, we analyze the transfer mechanisms for a set of catechols and resorcinols of a single electron, proton and hydrogen, with the radical peroxyl (˙OOH) and with different electron withdrawing and donating groups as substituents. By using the M05-2X exchange correlation functional within the Density Functional Theory framework combined with the 6-311++G(d,p) basis set, we were able to compute the Gibbs free energies for all mechanisms and compounds. According to the thermodynamic results, the hydrogen atom transfer mechanism was the most favorable. Therefore, this mechanism with substituents –CH3 and –COH in catechol and resorcinol was analyzed, using the reaction force and reaction electronic flux to characterize the structural and electronic changes that take place during the reaction. Our results show that electron donating groups favor electronic changes along the reaction path, increasing the spontaneity of the hydrogen atom transfer mechanism.


 Please wait while we load your content...
Please wait while we load your content...