Thionine–graphene oxide covalent hybrid and its interaction with light†
Abstract
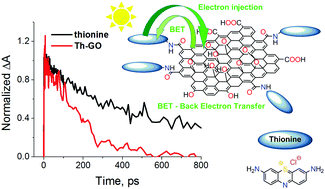
Graphene oxide sheets (GO) were covalently functionalized with thionine molecules. The obtained hybrid material, Th–GO, was characterized by means of scanning electron microscopy (SEM), Auger electron spectroscopy (AES), Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. Subsequently, the interaction of light with the free dye molecules and with dye molecules bound to the graphene oxide sheets was probed via UV-Vis spectroscopy, fluorescence spectroscopy and femtosecond pump–probe spectroscopy. The experimental results proved that thionine was successfully grafted onto the GO sheets, however, only one of the two amino groups of thionine was always involved in the amide bond formation. The Th–GO hybrid suspended in N,N-dimethylformamide (DMF) exhibited suppressed fluorescence as compared to the free dye in the same solvent, pointing to an efficient interaction between the photoexcited dye and the graphene sheets. Yet, no electron transfer products were detected by transient absorption measurements, even though there was a shortening of the singlet excited state lifetime of thionine (from the 567 ps for the free dye to the 313 ps for the dye in Th–GO). These results can be rationalized in terms of a fast back electron transfer process or possibly an energy transfer process.


 Please wait while we load your content...
Please wait while we load your content...