Single-layer MoS2 formation by sulfidation of molybdenum oxides in different oxidation states on Au(111)
Abstract
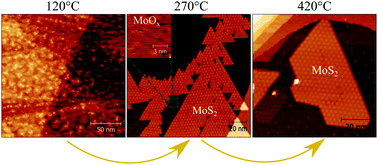
The sulfidation of a MoO3 precursor into MoS2 is an important step in the preparation of catalysts for the hydrodesulfurization process that is widely utilized in oil refineries. Molybdenum oxides are also the most commonly used precursors for MoS2 growth in, e.g., the synthesis of novel two-dimensional materials. In the present study, we investigate the transformation of MoOx into MoS2 on a model Au(111) surface through sulfidation in H2S gas atmosphere using in situ scanning tunneling microscopy and X-ray photoemission spectroscopy. We find that progressive annealing steps of physical vapor deposited MoO3 powder allow us to control the stoichiometry and oxidation state of the precursor oxide. Subsequently, we investigate the sulfidation of the compounds ranging from pure low-oxygen Mo to fully oxidized MoO3 oxide sulfidation using two different methods. We find that the prerequisite for the efficient formation of MoS2 is that Mo stays in the highest Mo6+ state before sulfidation, whereas the presence of the reduced MoOx phase impedes the MoS2 growth. We also find that it is more efficient to form MoS2 by post-sulfidation of MoOx rather than its reactive deposition in H2S gas, which leads to rather stable amorphous oxysulfide phases.


 Please wait while we load your content...
Please wait while we load your content...