Understanding how cAMP-dependent protein kinase can catalyze phosphoryl transfer in the presence of Ca2+ and Sr2+: a QM/MM study†
Abstract
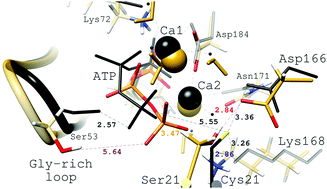
Recent experimental results have challenged conventional views on the role metals play in the chemistry of protein kinases because it has been shown that (cAMP)-dependent protein kinase (PKA) is active in the presence of other divalent alkaline earth metal cations besides physiological Mg2+ ions. This has raised the important possibility that Ca2+ may also be a physiological cofactor of protein kinases. In this work, QM/MM calculations, at the DFT and MP2 levels for the QM part, on complete solvated models of PKAc–M2ATP–substrate ternary complexes, with PKAc as the catalytic subunit of PKA, M denoting Ca2+ or Sr2+ and substrate denoting SP20 or Kemptide, have been carried out for the overall phosphoryl transfer reaction. In accordance with the experimental data, our theoretical results show for the first time at the molecular level how the overall PKAc-catalyzed phosphorylation of SP20, via a dissociative mechanism, is plausible with Ca2+ and Sr2+. The viability of the catalytic reaction with Kemptide and Ca2+ is also verified here. The energy barrier of the rate-limiting phosphoryl-transfer step does not depend on different coordination environments of the alkaline earth metal cations whereas the proton-transfer step region is metal dependent making the global chemical process more exoergic on going from Mg2+ to Sr2+. This trend is in agreement with the less effective release of the phosphorylated product observed experimentally in the presence of Ca2+versus Mg2+, and would explain also the lower activity of PKAc with Ca2+, since phospho-substrate and ADP releases are rate limiting for catalytic turnover. For the same reason, we predict an even lower activity of PKAc with Sr2+. Moreover, the active sites of the in silico reactant and product complexes and the available X-ray crystallographic structures show good agreement.


 Please wait while we load your content...
Please wait while we load your content...