Binding site opening by loop C shift and chloride ion-pore interaction in the GABAA receptor model†
Abstract
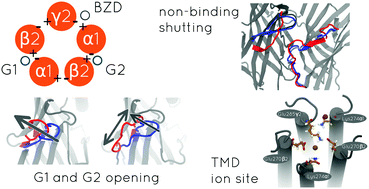
GABAA receptors (GABAARs) are crucial in mediating inhibition in the adult mammalian brain. Although the kinetics of this receptor has been extensively studied, the molecular picture of interactions occurring at various channel conformations remains elusive. While electrophysiology combined with mutagenesis sheds light on the role of specific residues, ultrastructural studies reveal static structures which, in the case of GABAARs, are limited to the β3 homomer. To take advantage of the newest crystal structures of cys-loop receptors, a homology model of α1β2γ2 GABAAR in the unbound closed state was built using a template of the homomeric glycine receptor in the closed state. The template model contained strychnine molecules at the binding sites which were removed and molecular dynamics was used to study the system relaxation. The modeled GABAAR preserved the closed conformation. Two interfaces forming orthosteric binding sites (β2/α1) exhibited opening due to the outward shift of loop C. Similar movement, although less pronounced, was observed at the α1/γ2 (modulatory) interface. In contrast, interfaces α1/β2 and γ2/β2 remained closed. The former one, due to interactions mediated mainly by loops C and F, affected the neighboring β2/α1 interface leading to asymmetry between the orthosteric binding sites. Such interactions were not observed at the β2/α1 interface preceded by a γ2 subunit. As expected, in the channel pore, the conserved leucine gate and selectivity filter were present. However, an additional constriction was found at the top of the pore which differed from a typical hydrophobic channel gate as it consisted of charged residues. Interestingly, this site showed a capacity to trap chloride ions and to undergo conformation transition-like expansion, suggesting an impact on pore properties. In conclusion, our homology model faithfully reproduced major features of heteromeric GABAARs offering insight into the underlying mechanisms of stabilizing the shut conformation and chloride ion interaction with the channel pore.


 Please wait while we load your content...
Please wait while we load your content...