The influence of substituents and the environment on the NMR shielding constants of supramolecular complexes based on A–T and A–U base pairs†
Abstract
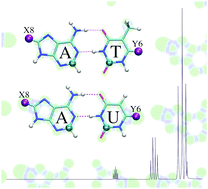
In the present study, we have theoretically analyzed supramolecular complexes based on the Watson–Crick A–T and A–U base pairs using dispersion-corrected density functional theory (DFT). Hydrogen atoms H8 and/or H6 in the natural adenine and thymine/uracil bases were replaced, respectively, by substituents X8, Y6 = NH−, NH2, NH3+ (N series), O−, OH, OH2+ (O series), F, Cl or Br (halogen series). We examined the effect of the substituents on the hydrogen-bond lengths, strength and bonding mechanism, and the NMR shielding constants of the C2-adenine and C2-thymine/uracil atoms in the base pairs. The general belief in the literature that there is a direct connection between changes in the hydrogen-bond strength and the C2-adenine shielding constant is conclusively rejected by our computations.


 Please wait while we load your content...
Please wait while we load your content...