The effect of hydroxyl on the solution behavior of a quaternary ammonium gemini surfactant†
Abstract
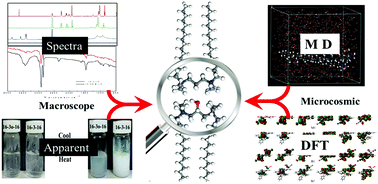
The adsorption and viscoelastic properties of a micellar solution of 2-hydroxyl-propanediyl-1,3-bis(hexadecyldimethylammonium bromide), abbreviated as 16-3OH-16, have been investigated by surface tension and rheological measurements. Meanwhile, an aqueous solution of propanediyl-1,3-bis(hexadecyldimethylammonium bromide), abbreviated as 16-3-16, was also examined. From the steady state and oscillatory rheological results, a notable difference in shear viscosities between the two systems was observed. Zeta potentials and size distributions confirm the change in the potentials and hydrodynamic diameters, and these results are in good agreement with the rheological results. The differences of the two solutions were attributed to the effect of the hydroxyl group on the spacer of 16-3OH-16. Molecular dynamic simulations and density functional theory (DFT) calculations were performed to investigate the non-covalent interactions in the solution and the difference between the molecular orbitals and the electrostatic potentials. Our research shows that a more uniform distribution of positive charges around the spacer could result in a more effective electrostatic screening effect between the charged headgroups, and promote the formation of a worm-like micelle. Also, hyperconjugation becomes stronger when the hydroxyl group is introduced on the spacer of the gemini molecule.


 Please wait while we load your content...
Please wait while we load your content...