Effects of electrostatic interaction on the properties of ionic liquids correlated with the change of free volume
Abstract
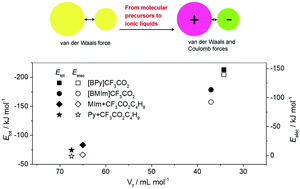
In this study, the amount of free volume in ionic liquids (ILs) was calculated and found to be almost half to that of their isoelectronic neutral analogues. MD simulations revealed that the significantly compressed free volume in the ILs was dominantly attributed to the strong inter-ion electrostatic interactions, which are comparable to the application of an external pressure of ∼250 MPa to the neutral analogues. Furthermore, the change in the free volume shows an interconnection with other properties of ILs, especially viscosity. The inherent high viscosity of ILs was quantitatively correlated to a low free volume available for mass transfer, and the sharp decrease in the viscosity of ILs with the addition of organic solvents was essentially caused by the introduction of free volume.


 Please wait while we load your content...
Please wait while we load your content...