Kinetics of ozone decomposition in porous In2O3 monoliths†
Abstract
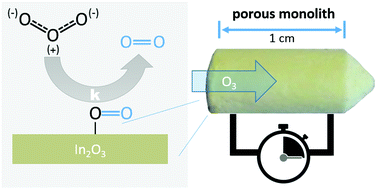
The performance of many chemical gas-phase reactions is strongly influenced by the interaction of reactants with interfaces. Nanoporous materials, which exhibit pore diameters up to 100 nm and high specific surface areas, can be utilized to reduce the amount of cost-intensive materials (e.g. noble metals). However, due to limitations in material transport and reaction kinetics detailed knowledge of the diffusion and the kinetics of a chemical reaction is necessary to improve the performance of chemical processes in industry and research. To experimentally study the diffusion and reaction kinetics of gaseous species inside such pores, the chemoresistive behavior of certain metal oxides such as In2O3 can be utilized. In this work, we present a model system based on hierarchically porous monolithic indium oxide (In2O3) which allows the determination of kinetic effects by utilizing its gas transducing properties. The experimental data obtained by electrical measurements are compared to two diffusion and diffusion–reaction models. Using these methods, the rate constant of ozone decomposition in porous In2O3 is estimated. The results are the basis for a suitable material design for semiconducting gas sensors, on the nano-, meso- and macroscale, which helps in understanding the underlying mechanisms of diffusion and reaction.


 Please wait while we load your content...
Please wait while we load your content...